25 ++ periodic table with solubility rules 112704-Periodic table with solubility rules
3) if there is more solute than is able to be dissolved, the excess solute separates from theAug 15, · Depending on the solubility of a solute, there are three possible results 1) if the solution has less solute than the maximum amount that it is able to dissolve (its solubility), it is a dilute solution;Solubility rules state that all hydroxide compounds are insoluble in water, except sodium and potassium hydroxide Calcium hydroxide is water insoluble and could possibly be the identity of base A Recall that sodium and potassium are alkali metals (column I of periodic table);

Solubility Factors When Choosing A Solvent Labclinics
Periodic table with solubility rules
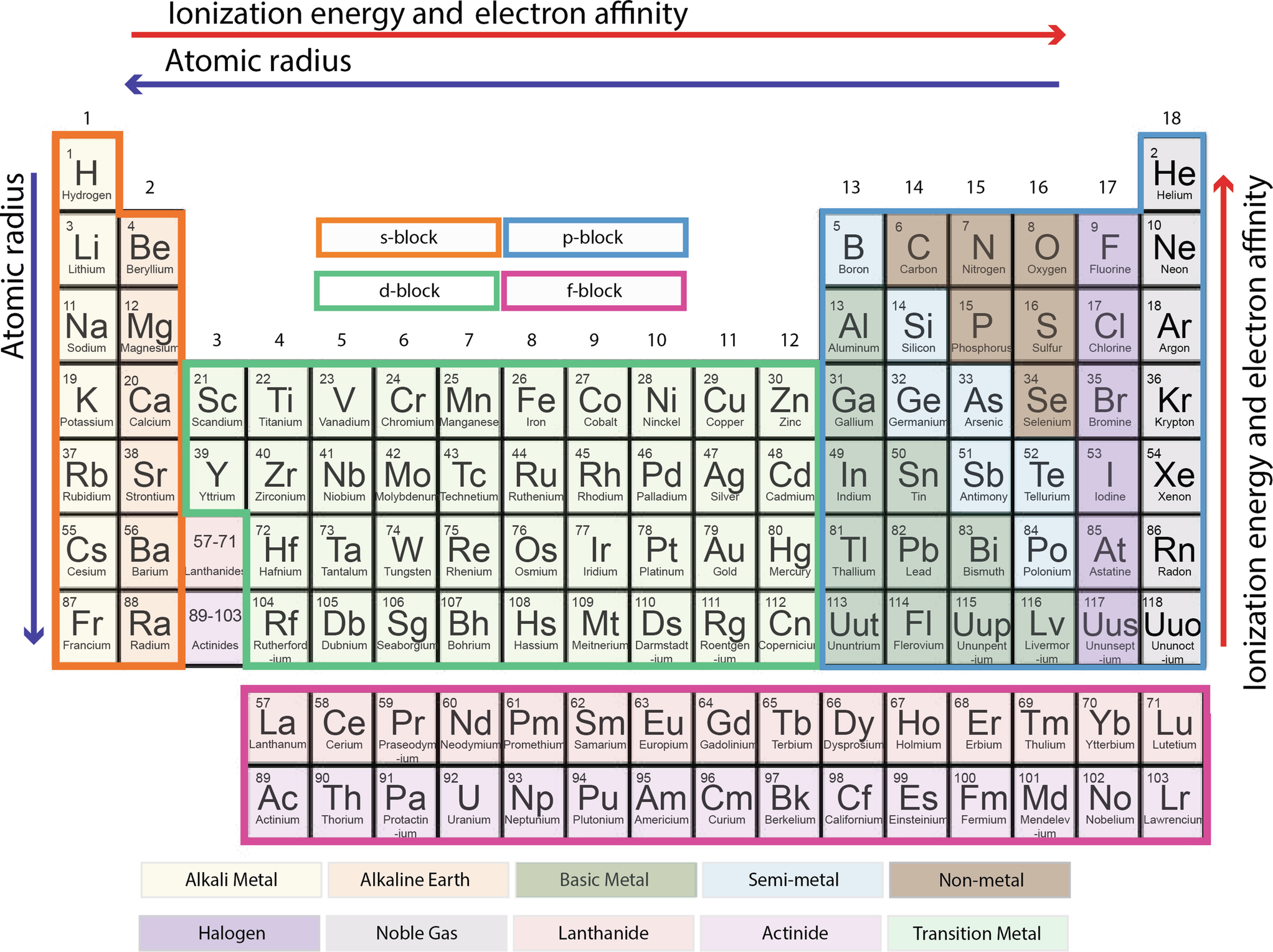
Periodic table with solubility rules-Aug 13, · 57 Predicting Solubility Trends The solubility of many simple ionic compounds can be predicted by applying the set of rules shown below Salts of the alkali metal ions and the ammonium ion, Li , Na , K , and NH 4 are almost always soluble Virtually all metal nitrates and metal acetates are soluble Metal halides are generally solublePeriodic Table Interactive Royal Society of Chemistry (updated 17) Interactive American elements interactive Chemical elements NOTE The following site was made by an 8th grader over a decade ago So not updated, but amazing Great site for investigating elements but always use multiple websites when researching properties of elements



Precipitation Reactions Pathways To Chemistry
The Solubility Table / Chart allows us to solubility of substances in water This is useful for chemistry topics such as net ionic equations or predicting whGet an answer for 'Describe the relationship between the solubility of the salts containing alkaline earth metal ions and the position of the metals in the periodic table' and find homework helpE2, CHM111, F Name____HARBI OLHAYE Important Use the periodic table provided in this exam Solubility rules and rules for the oxidation number are at the end of this exam Q1 A compound contains C, H, and N only Analysis of a sample of the compound showed that it contained 386 % C, 164 % H and the rest Nitrogen The molar mass of the compound is 62 g/mole
Oct 09, 19 · 2 KCl (aq) Pb (NO 3) 2 (aq) → 2 KNO 3 (aq) PbCl 2 (s) The solubility rules are a useful guideline to predict whether a compound will dissolve or form a precipitate There are many other factors that can affect solubility, but these rules are a good first step to determine the outcome of aqueous solution reactionsThis video discusses the solubility rules by showing the relationship between the table of solubility rules respectively toward the perodic tableTherefore, these elements have one electron in its outermost
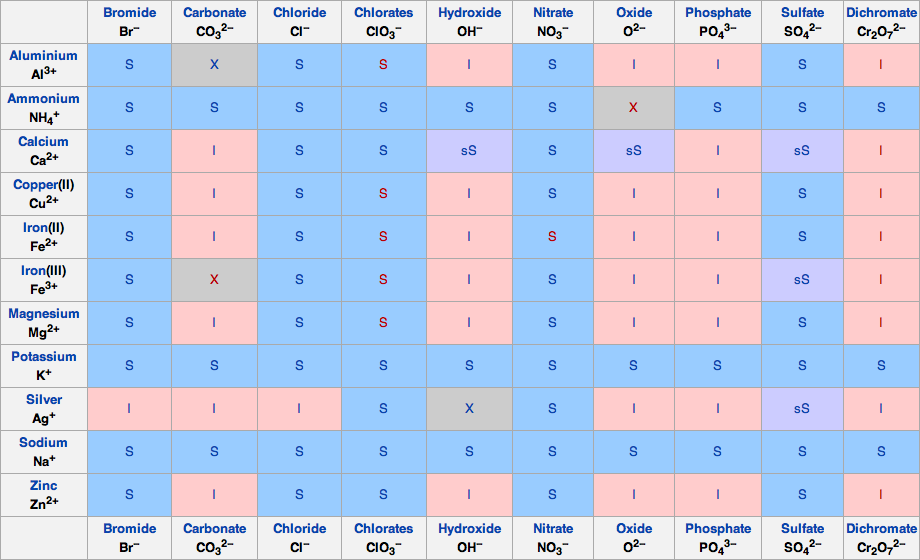
You might find this easier Cations are listed across the top, and anions are listed vertically Find the cell where your cation column and ion row meet to determine solubility of the resulting compound Our solubility rules are not exhaustive You may need to reference a periodic table if you're looking up less common compounds SolubilityNov , 18 · Use the solubility rules and periodic table to predict the product that will precipitate out in the reaction 1 See answer chad55 is waiting for your help Add your answer and earn points tallinn tallinn Answer E Explanations The reactants areNov 05, 11 · VIDEO Solubility Table Examples 1 PRACTICE PROBLEMS Determine if the chemical is INSOLUBLE or SOLUBLE and give what state it would be marked with in a chemical equation (aq or s) Make sure to use the solubility table and precipitation definitions if you need them Another useful tool is the ion periodic table




Chapter 4 Section 2




The Periodic Table Protocol
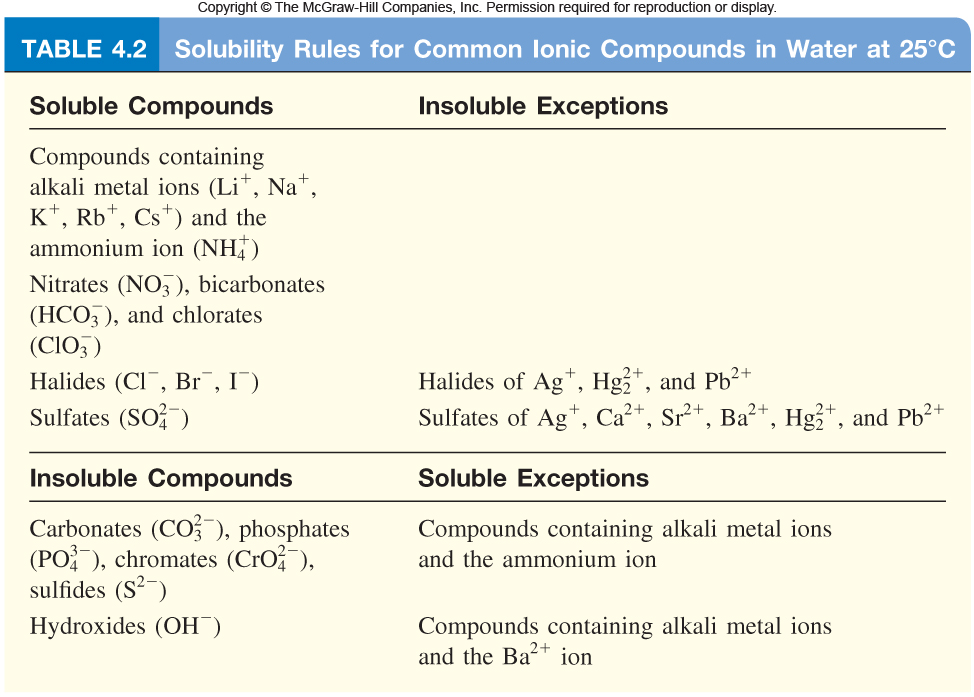
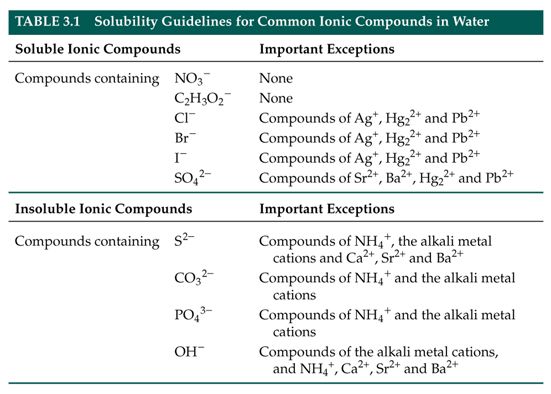
Table 41 "Some Useful Solubility Rules" lists some general solubility rules We need to consider each ionic compound (both the reactants and the possible products) in light of the solubility rules in Table 41 "Some Useful Solubility Rules" If a compound is soluble, we use the (aq) label with it, indicating it dissolvesThus, is soluble in aqueous solutionSOLUBILITY RULES Most of the precipitation reactions that we will deal with involve aqueous salt solutions Remember salts are compounds which consist of metal cations like Na , Ca 2, Cu 2 (or the one nonmetal molecular ion that we have discussed, ammonium NH 4 ) ionically bonded to nonmetal anions such as Cl, (including molecular anions such as hydroxide OH, sulfate



Http Www1 Udel Edu Chem Sametz 101fall09 Ch4 Pdf




Solubility Table Easy Hard Science
2) if the amount of solute is exactly the same amount as its solubility, it is saturated;Elements on the periodic table solubility rules a set of rules used to predict whether an ionic compound will be soluble or insoluble in water Use the solubility rules in Table 194 to determine whether these compounds are soluble or insoluble a NH 4 OH b Ca(NO 3Unit 4 Periodic Table Handouts color coded periodic table blank periodic table Unit 5 Chemical Formulas Handouts Elements to Know for the Element name Test fat daddy page 1 fat daddy page 2 fat daddy page 3 fat daddy page 4 flow chart of nomenclature oxidation rules



1




Implementing Solubility Rules In The Periodic Table Youtube
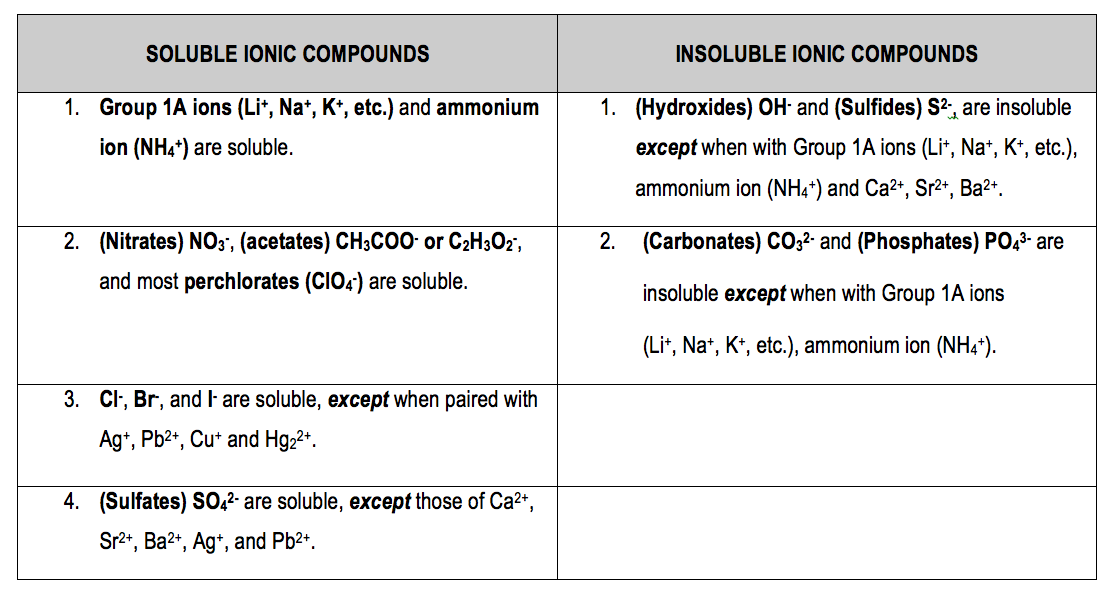
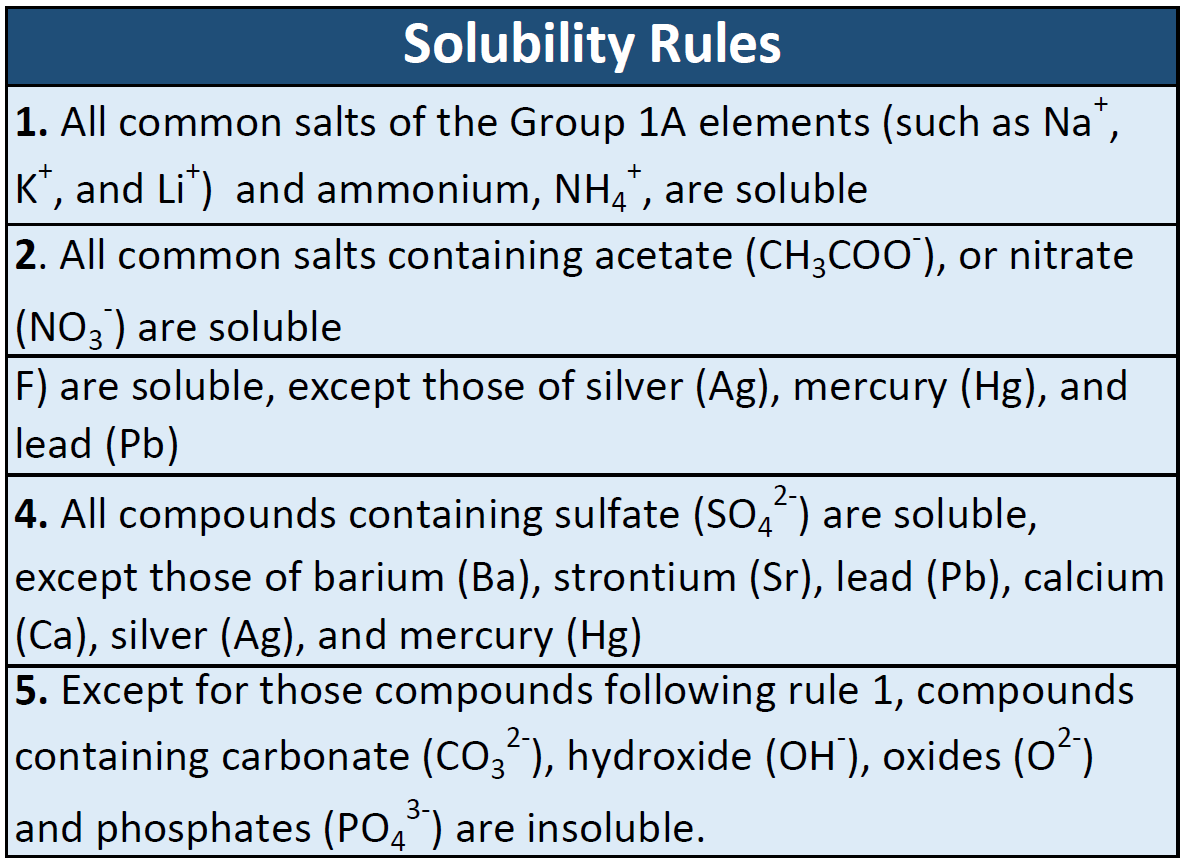
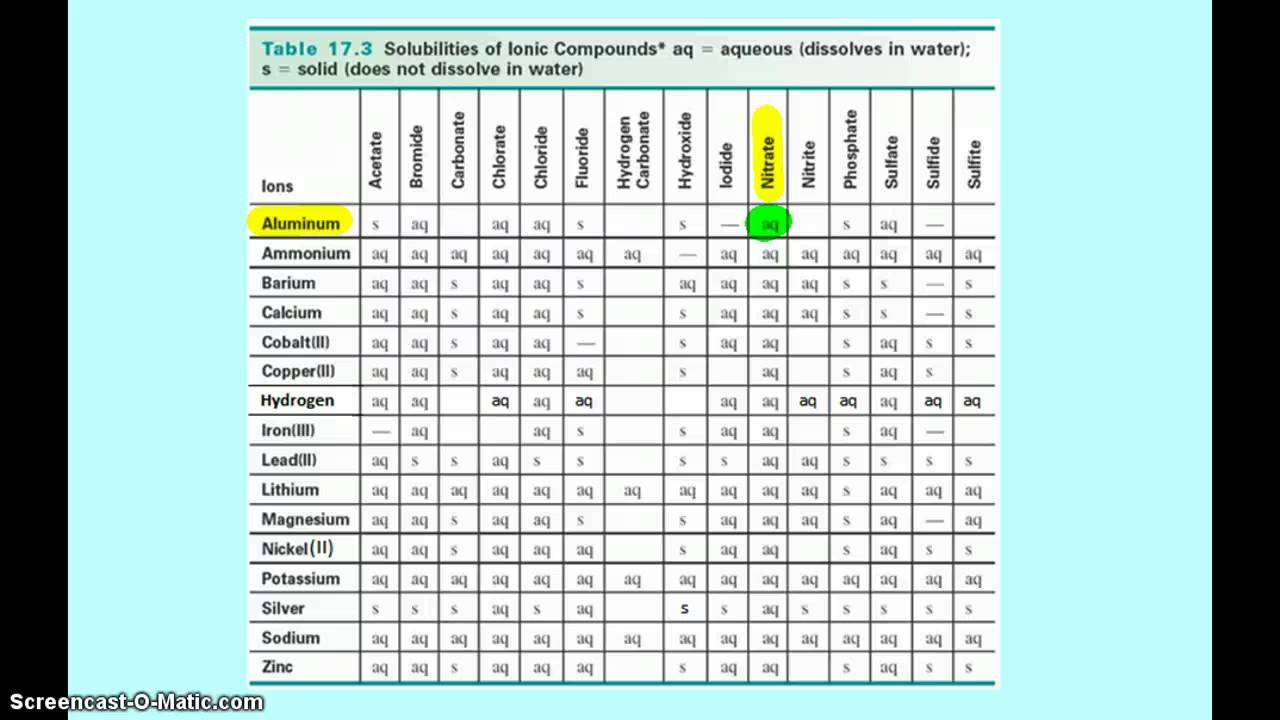
Jun 07, 21 · Rules for naming ionic compounds containing polyatomic ions Name the cation ( ion) first (watch for nh 4 ammonium) 2 • use a periodic table of elements and of ions Nano3 sodium nitrate na2so4 sodium sulfate cacl2 calcium chloride k3po4 potassium phosphate For binary ionic compounds (ionic compounds that contain only two types ofThis is the solubility table that is available for use within ALEKS Feel free to have this table with you when you take the assessment or when you are in learning mode in ALEKS Even so, you will save a lot of time (now and later in UT Chem classes) if you commit these two solubility rules to memory • Compounds with Group 1 cations and NH 4Solubility Rules and Common Ions Solubility Rules NO31 All nitrates are soluble Cl1 All chlorides are soluble except AgCl, PbCl2, and Hg2Cl2 SO42 All sulfates are soluble except Ag2SO4, PbSO4, Hg2SO4, CaSO4, SrSO4, and BaSO4 CO32 The carbonate of Group 1 metals and (NH4)2CO3 are soluble All other carbonates are insoluble




Types Of Chemical Reactions Single And Double Displacement Reactions Introductory Chemistry



Periodic Table Springerlink
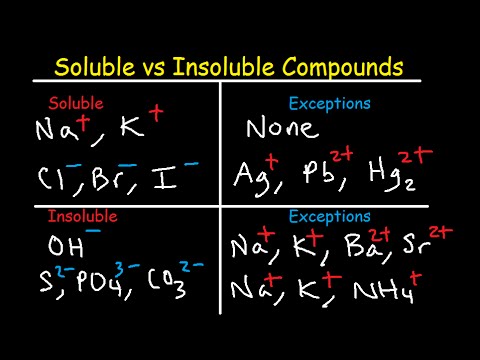
Pivot Interactives Solubility Rules Answers / Ammonium On Periodic Table Elcho Table / Looking to be able perform advanced pivoting in r, using various functions and slicers • solubility rules • masses of gases pivot interactives Create interactive pivot table using pivottablejsReferencing the periodic table below will help you work through the elemental names and groups Salts containing Group I elements (Li, Na, K, Cs, Rb) are soluble There are few exceptions to this rule Salts containing the ammonium ion (NH4) are also solubleSOLUBILITY RULES Solids (Precipitates) COMPOUND CONTAINS GENERAL SOLUBILITY EXCEPTIONS EXAMPLES Li, Na, K, NH 4 Always Soluble None NaBr, K 2SO4, (NH4)2CO3 are soluble NO3, C2H3O2 (Nitrates and Acetates)



Superior Periodic Table With Ion Chart Solubility Chart By Dave Swaney




Dr Starkey S Chm 121 General Chemistry
Nov 19, 19 · Solubility Rules All salts of the group I elements (alkali metals = Na, Li, K, Cs, Rb) are soluble NO 3 All nitrates are solubl e Chlorate (ClO 3), perchlorate (ClO 4), and acetate (CH 3 COO or C 2 H 3 O 2, abbreviated as Oac ) salts are solubleCommit to memory the solubility rules required for this course (see below) You will be expected to know these throughout the year Rules for the solubility of salts in water 1 Salts containing the alkali metal (group 1A) ions and the ammonium ion are soluble Exceptions to this rule are rare 2The formation of a precipitate, when two solutions are mixed, can be predicted using the solubility rules in the table if the products meet the 'soluble' rules in the left hand column, no



Periodic Table Chemistry Reference Sheet By Mike Stratton Tpt



Use The Solubility Rules To Determine Whic Clutch Prep
Use the periodic table, an activity series, or solubility rules to predict whether singlereplacement reactions or doublereplacement reactions will occur Up to now, we have presented chemical reactions as a topic, but we have not discussed how theInteractive periodic table with uptodate element property data collected from authoritative sources Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game!Solubility Parameter Search (SP Value, Chemical) Solubility parameter (SP value) is an application for searching the SP value of a chemical substance that is an index of solubility This app is currently available in Japanese and English SP value can be searched by CAS number and chemical name (Japanese / English)



Search Q Solubility Periodic Table Trend Tbm Isch



Chemistry Part 6
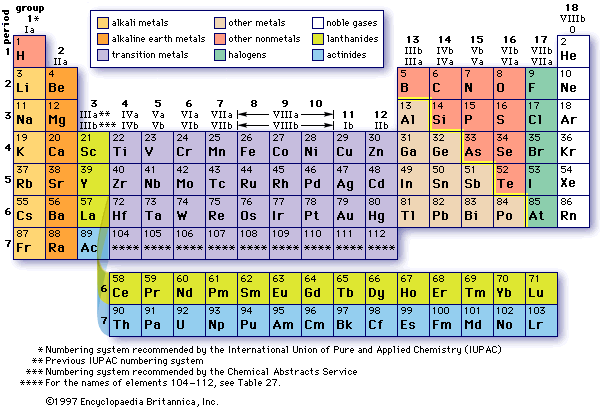
Periodic Table Packet W21pdf Simple solubility rules Applies to Li Na K Cs Rb NH4 Acetate(C2H3O2\u13 NO3\u13 Cl\u13 Br\u13 I\u13 Rule Soluble Soluble Periodic Table Packet W21pdf Simple solubility rulesTable 41 "Some Useful Solubility Rules" lists some general solubility rules We need to consider each ionic compound (both the reactants and the possible products) in light of the solubility rules in Table 41 "Some Useful Solubility Rules" If a compound is soluble, we use the (aq) label with it, indicating it dissolvesMar 05, · The modern Periodic Table is based on an earlier Periodic Table made by a scientist called Mendeleev Others had tried to arrange all the elements before, but Mendeleev was the first to correctly arrange it by both Relative Atomic Mass and chemical properties with gaps where elements were later discovered
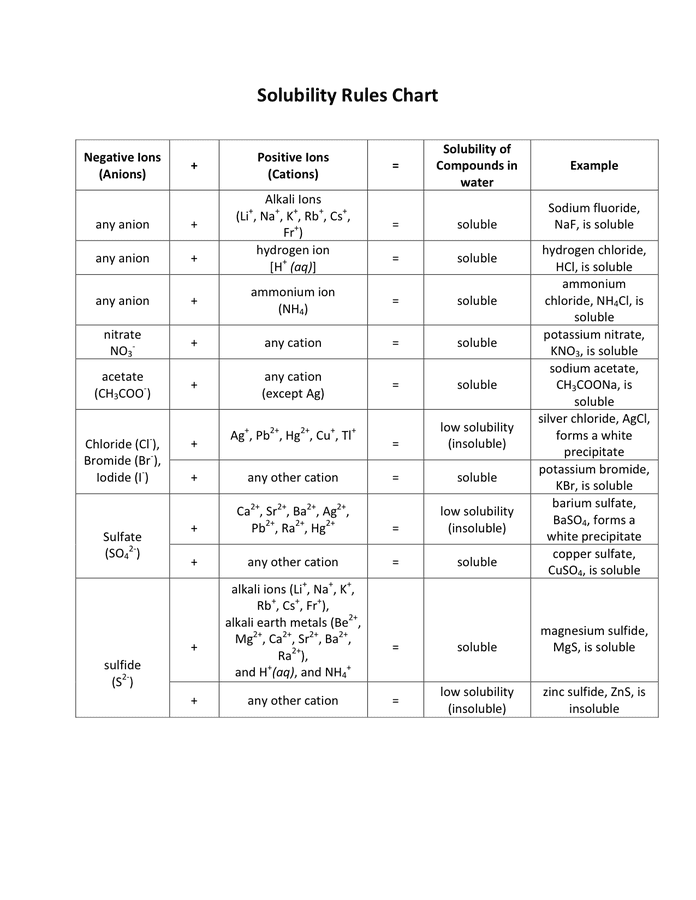



Solubility Rules Chart In Word And Pdf Formats




Chapter 9 Aqueous Solutions Predicting Solubilities Solubility Chemists
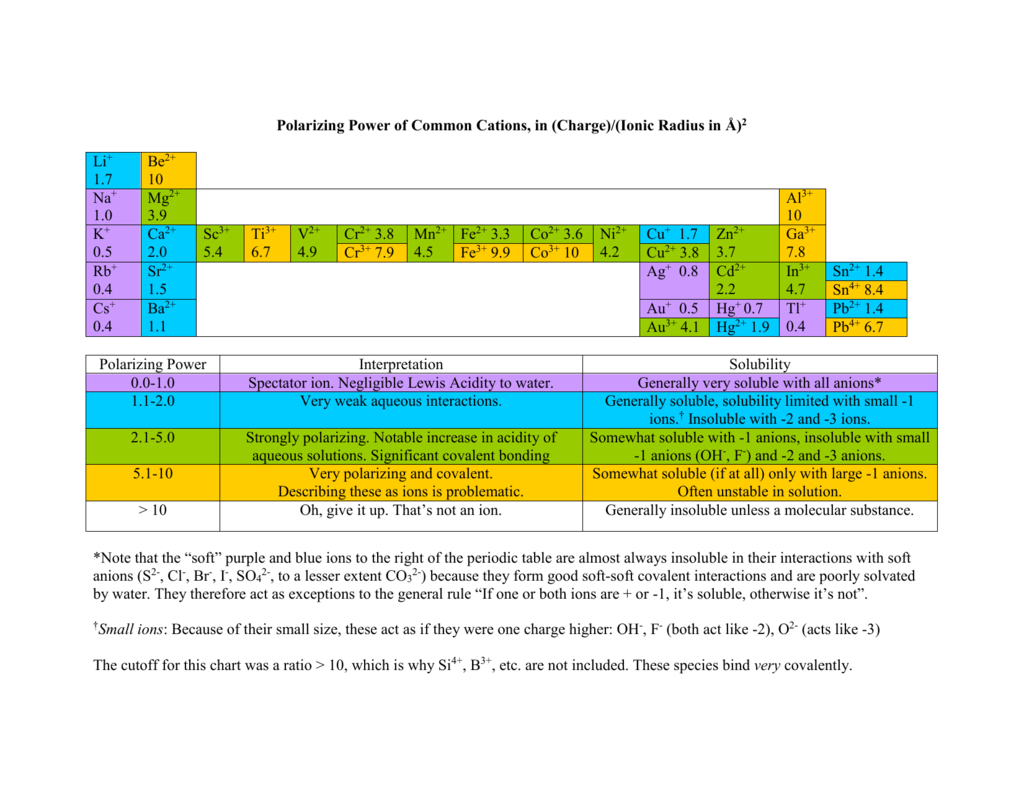
Feb 05, 18 · Learning the Solubility Rules Recognize that salts containing Group 1A elements are soluble The periodic table is organized into rows and columns called periods and groups, respectively The first column of the table contains the Group 1A elements They are the alkali metals and include Li, Na, K, Cs, and RbQuestion 12 State a general rule that relates the solubility of an ionic compound with the charges on the ions of which it is composed Question 13 In your Data Table A, write the chemical formula for any compound that precipi4 Propose an explanation for the observed solubility pattern The size or radius of an atom increases as one goes down a row (family) in the periodic table For cations, the charge density (charge per unit volume) will therefore decrease going down a row The solubility of metal cations in water is strongly influenced by the hydration



Tables Of Chem
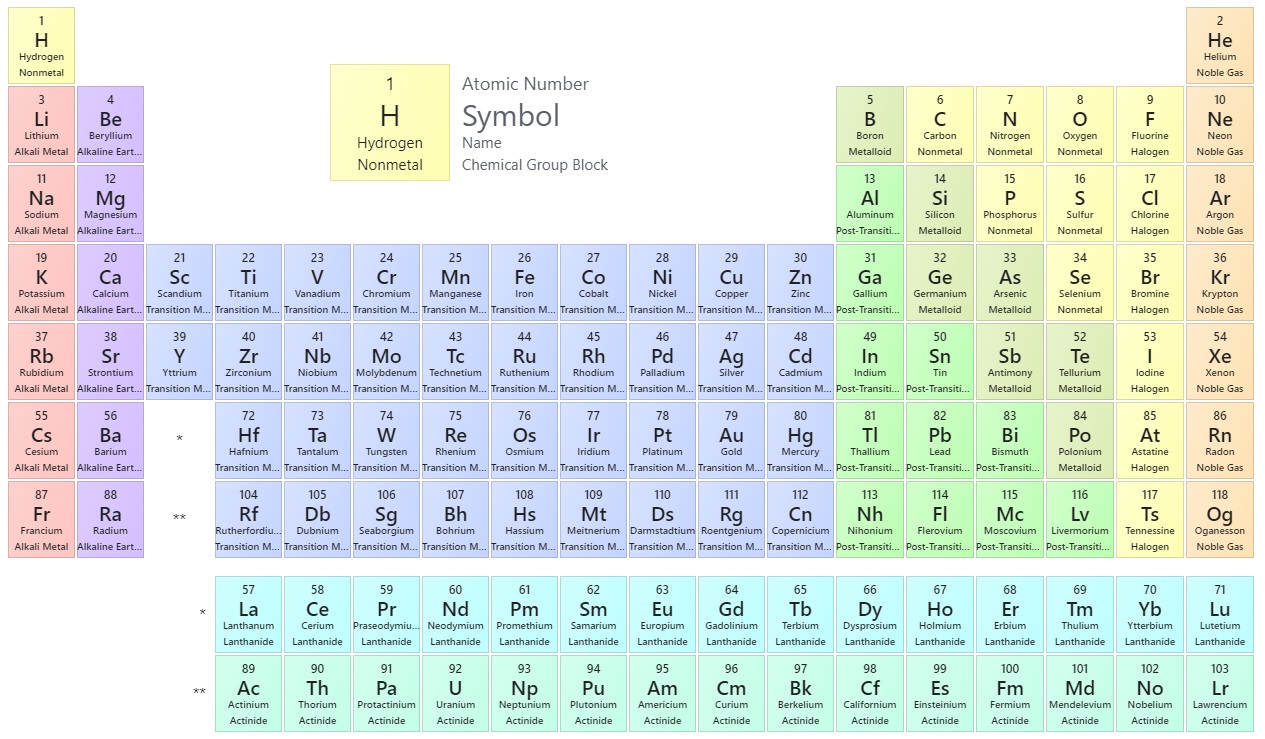



4 6 Looking For Patterns The Periodic Law And The Periodic Table Chemistry Libretexts
The Periodic Table for Chemistry A time line at the bottom highlights the ongoing nature of chemical understanding, and places some of the more critical developments of the periodic table in historical perspective The back has a chart to find electronegativity and oxidation number values, a list of solubility rules, nomenclatureSolubility rules can help us find the answer to this question First, solubility rules state that all compounds of Group 1A elements on the periodic table are soluble in aqueous solution This means that all of the alkali metals, including potassium, form compounds which are soluble in aqueous solution;A Table for the Solubility of Salts in Water Brief Introductory Discussion These rules are general and qualitative in nature Each rule has exceptions and the important ones are noted The most common definition for solubility is this 1) Soluble substances can form a



Chem Precipitation Rules Solubility Table Scientific Tutor
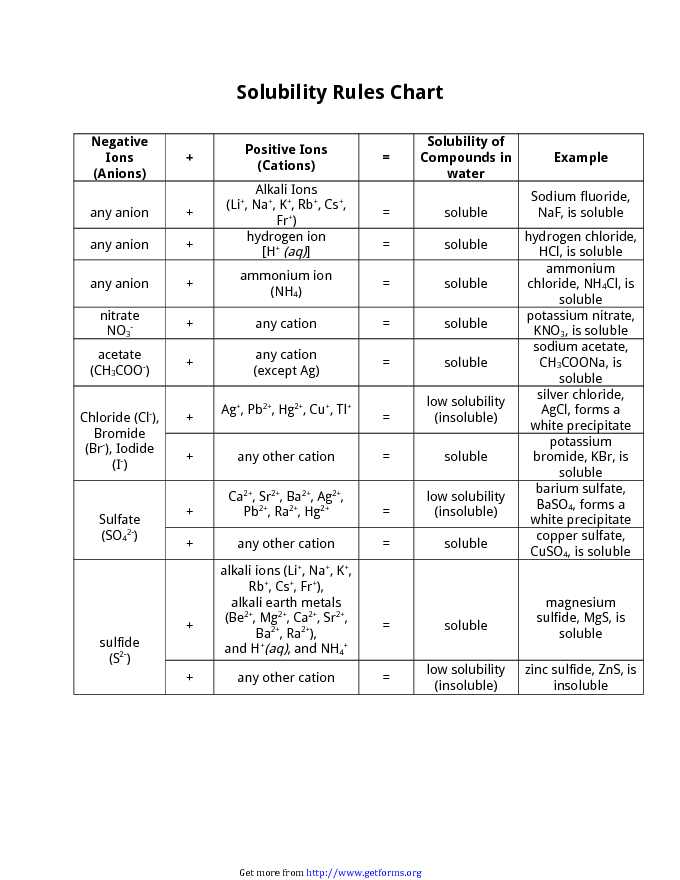



Solubility Rules Chart Download Chemistry Chart For Free Pdf Or Word
Advanced application of Periodic table The newest and most reliable information on the elements Interactive and userfriendly interface The possibility of studying the gaming table Chart of solubilityQuestion 12 State a general rule that relates the solubility of an ionic compound with the charges on the ions of which it is composed Question 13 In your Data Table A, write the chemical formula for any compound that precipiQuestion 11 Considering the general rules you found for Group 1 ions and phosphate ion, which rule takes precedence?



Chemistry Periodic Table




Use The Solubility Rules And The Periodic Table To Predict The Product That Will Precipitate Out In Brainly Com
Nov , · Solubility Guidelines* *Pertains to substances in water at room temperature and standard pressure Salts containing Group I elements (Li , Na , K , Cs , Rb ) or ammonium ions (NH 4) are soluble Salts containing nitrates (NO 3), acetates (C 2 H 3 O 2), chlorates (ClO 3), and perchlorates (ClO 4) are generally solubleThe solubility pattern observed for alkaline earth metal compounds is shown below The solubility of alkaline earth metal compounds decreases as you go down the column in the periodic table, ie, solubility decreases as the atomic mass of the alkaline A1 B1 C1 D1 B2 C2 D2 A3 C3 D3 B4 C4 D4 A5 B5 C5 D5 A6 B6 C6 D6 IO 3 – SO 4 2– C 2O 4 CO 3 2– control MgCl 2 CaCl 2 SrCl 2Table 2 states that K sp for Ag 2 CO 3 is 85 × 10 –12 The solubility equation involves the square of Ag because each formula unit yields two ions of Ag Because the molarity of is the same as the overall molarity of Ag 2 CO 3 in the solution, call the carbonate concentration x and the silver ion concentration 2 x



Cem1008f Periodic Table Solubility Rules And Formula Sheet Studocu



Solubility Table Sayre Chemistry 2
Solubility Rules Table The solubility classification of ionic substances according to their solubility in water is diffi cult Nothing is completely "insoluble" 'in water The degree of solubility varies from one "soluble" substance to another Nevertheless, solubility classification schêrnePowered by Create your own unique website with customizable templates Get StartedQuestion 11 Considering the general rules you found for Group 1 ions and phosphate ion, which rule takes precedence?




The Bio Relevant Metals Of The Periodic Table Of The Elements



1
Then, by carrying out the indicated reactions or possible reactions, omitting those shaded blocks, and recording your observations, you should be able to generate or formulate a tentative list of solubility rules Use the Periodic Table to locate various positive ions, so that you can refer to them as Group I or II whenever generalization isUnderstanding solubility rules Water is commonly used as a solvent in solutions When a substance is soluble in water, it is said to be aqueous The notation (aq) is used for the physical state and specifically means that substance will dissolve in water Solubility rules for ionic compounds are on your Periodic Table




An Interdisciplinary Perspective From The Earth Scientist S Periodic Table Similarity And Connection Between Geochemistry And Metallurgy Sciencedirect



Precipitation Reactions Pathways To Chemistry




Chem 210 Periodic Table Solubility Rules And Naming Diagram Quizlet



Ch104 Chapter 7 Solutions Chemistry




Tips To Survive Grade 11 Chemistry




Solubility Chart And Solubility Rules Youtube



Solubility Chart



Soluble And Insoluble Compounds Chart Solubility Rules Table List Of Salts Substances Youtube




Periodic Table For General Chemistry Exam Edition White Amazon Com Industrial Scientific




Solubility Factors When Choosing A Solvent Labclinics
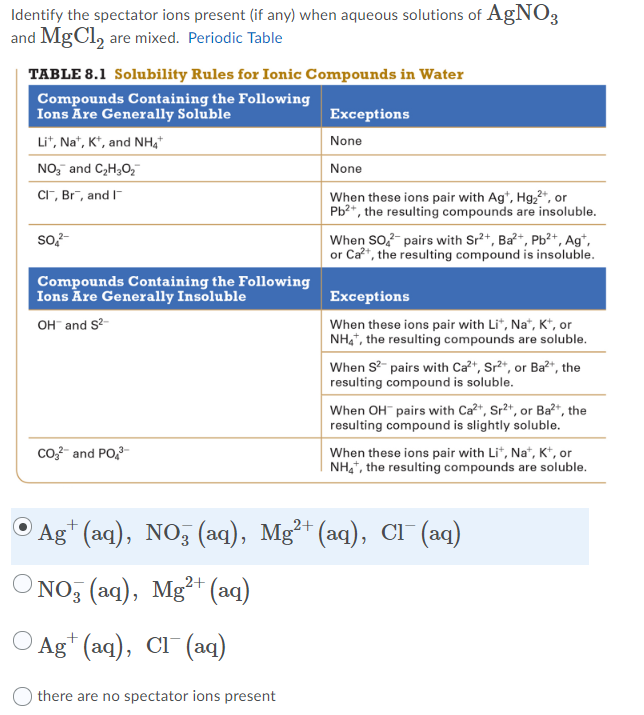



Identify The Spectator Ions Present If Any When Chegg Com
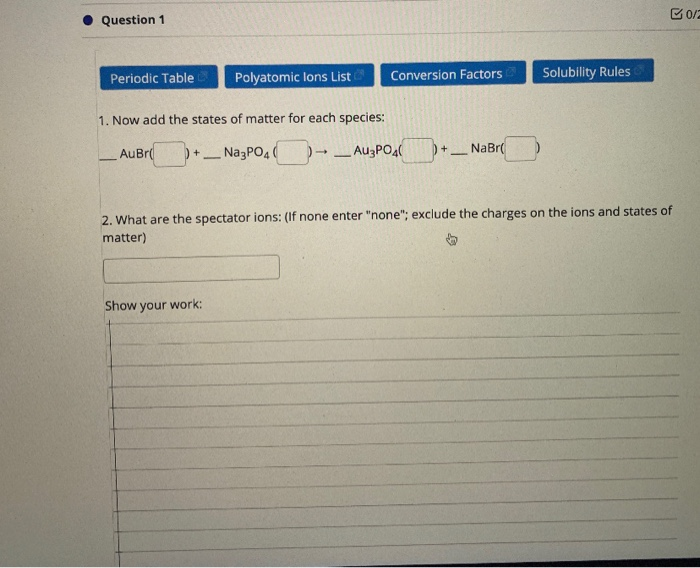



Solved Question 1 Go Periodic Table Polyatomic Lons List Chegg Com




Use The Solubility Rules And Periodic Table To Predict The Product That Will Precipitate Out In The Brainly Com
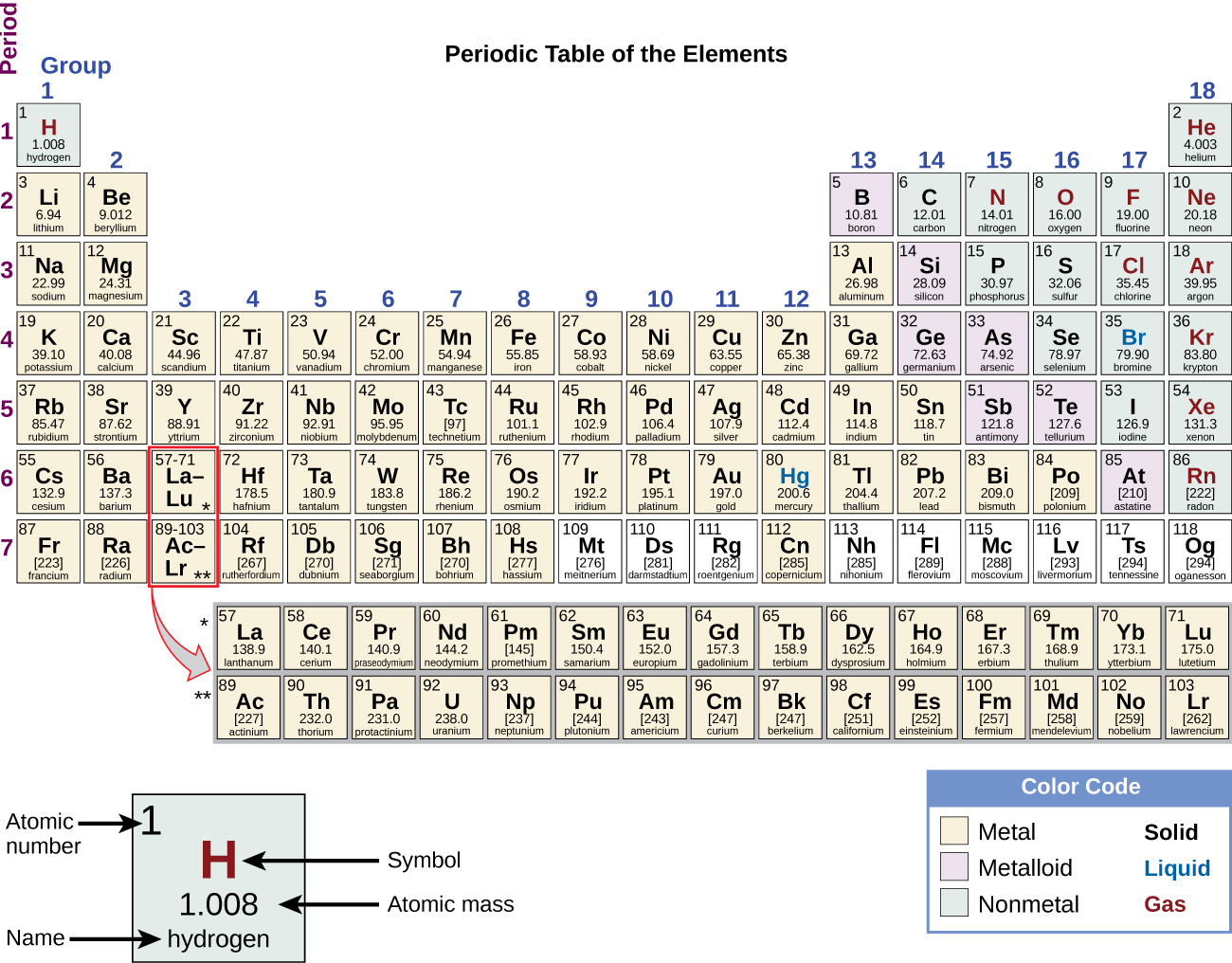



The Periodic Table Chemistry Atoms First 2e




ป กพ นในบอร ด Tutoring




Solubility Rules Flashcards Quizlet




Solubility Rules Solubility Of Common Ionic Compounds
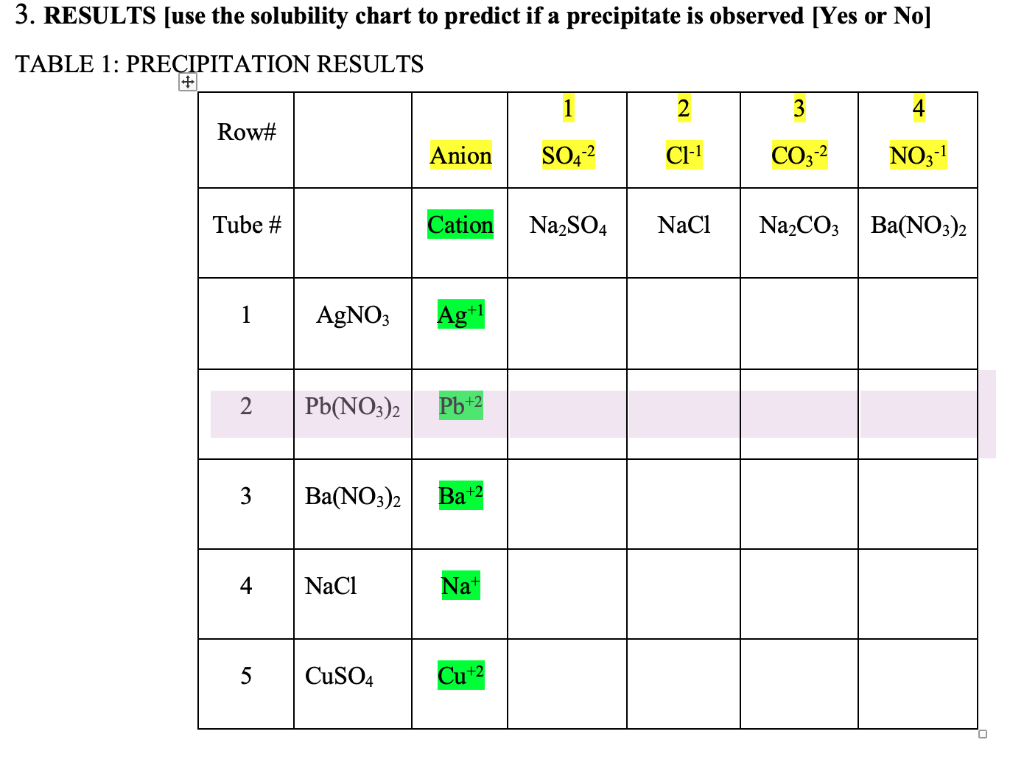



Table 5 1 Rules For Determining The Solubility Of Chegg Com




Solubility Rules The Ultimate Guide To Ap Chemistry Albert Io



Solubility Rules Sayre Chemistry 2




Precipitation Reactions 10 Or Some Solubility Rules For Ionic Compounds In Water Are Shown Homeworklib



Http Www1 Udel Edu Chem Sametz 101fall09 Ch4 Pdf



6 6 Types Of Chemical Reactions Single And Double Displacement Reactions Chemistry Libretexts



Public Domain Databases Of Interest To Scientists




Location




Solubility Rules Ppt Download




Chemistry Lab Periodicity Wiki Scioly Org



Using A Solubility Table Youtube




Mini Video On Ion Size Video Khan Academy



The 11 Solubility Rules And How To Use Them




Solubility Lovejoy Pre Ap Chemistry




Periodic Table Wikipedia




Alkali Metal Definition Properties Facts Britannica




How To Determine The Chemical State Of A Reactant Or Product In A Chemical Equation Chemistry Stack Exchange



Which Of The Following Substances Are Insoluble In Water Socratic




Chemistry Chart Periodic Table Of The Elements In Word And Pdf Formats Page 2 Of 2




Solubility Table Easy Hard Science




Holloway High School



Vitamins And Minerals




Chemistry The Central Science Chapter 4 Section 2



Http Www Ars Chemia Net Permanent Files Tables Periodic Table Pdf



Www Sewanhakaschools Org Cms Lib Ny Centricity Domain 25 16 reference tables Pdf



The Periodic Table Chemistry 2e




Solubility Rules Worksheet As You Work Through The Webassign




Chem 1180 17 1 The Solubility Product Constant




Ii Periodic Table




Hd Periodic Table Wallpaper Muted Colors



Groups Of The Periodic Table Video Khan Academy




Chem 1180 17 1 The Solubility Product Constant




Mercury Definition Uses Density Facts Britannica



What Are The Products For The Following Reaction Na 3po 4 Cacl 2 Socratic




Unit 10 Energy In Chemical Reactions Cypress Creek High School Chemistry 1k Chapter Ppt Download



Chem Precipitation Rules Solubility Table Scientific Tutor



Polarizing Power Of Common Cations



Chem 123 General Chemistry 1



Solubility Of Liquids
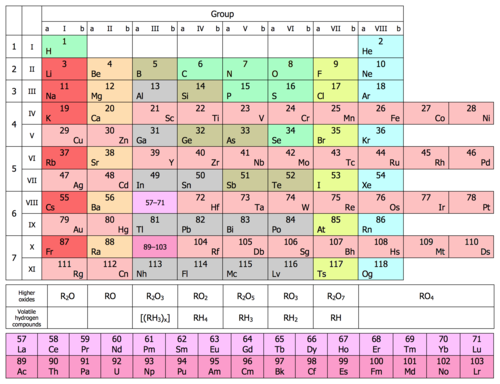



Periodic Table Wikipedia




Ward S Advanced Periodic Table Vwr



Double Replacement Reactions 1 The Objective Of This
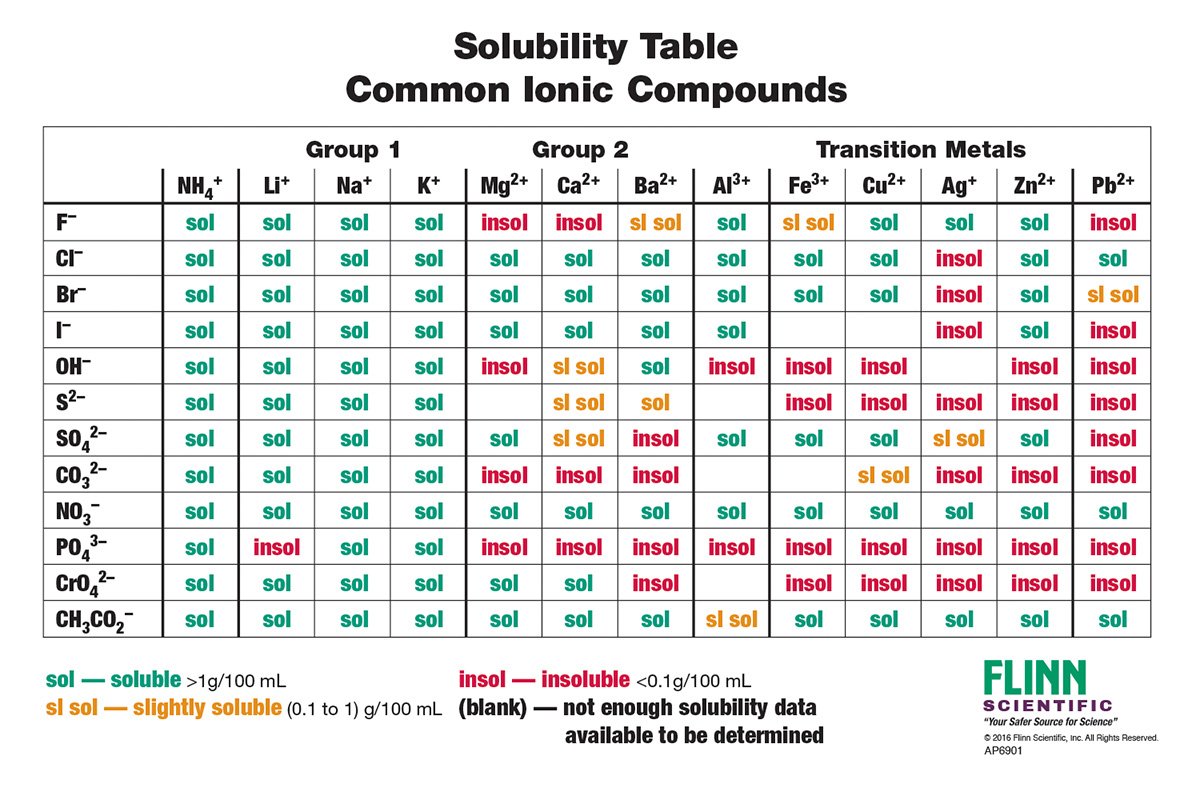



Solubility Rules Chart




3 Ways To Memorize The Solubility Rules For Common Ionic Compounds In Water




501 50 X 38 Intermediate Periodic Table Each Amazon Com Industrial Scientific




Minilab 5 03 Solubility Rules Mini Labs Org




Solubility Rules Ppt Download



The Periodic Chart Of Table Of The Elements Wyzant Lessons



Alternative Periodic Tables Updated Now With A Final Thought Chemistry Blog



The Hume Rothery Rules For Solid Solution Materials Science Engineering
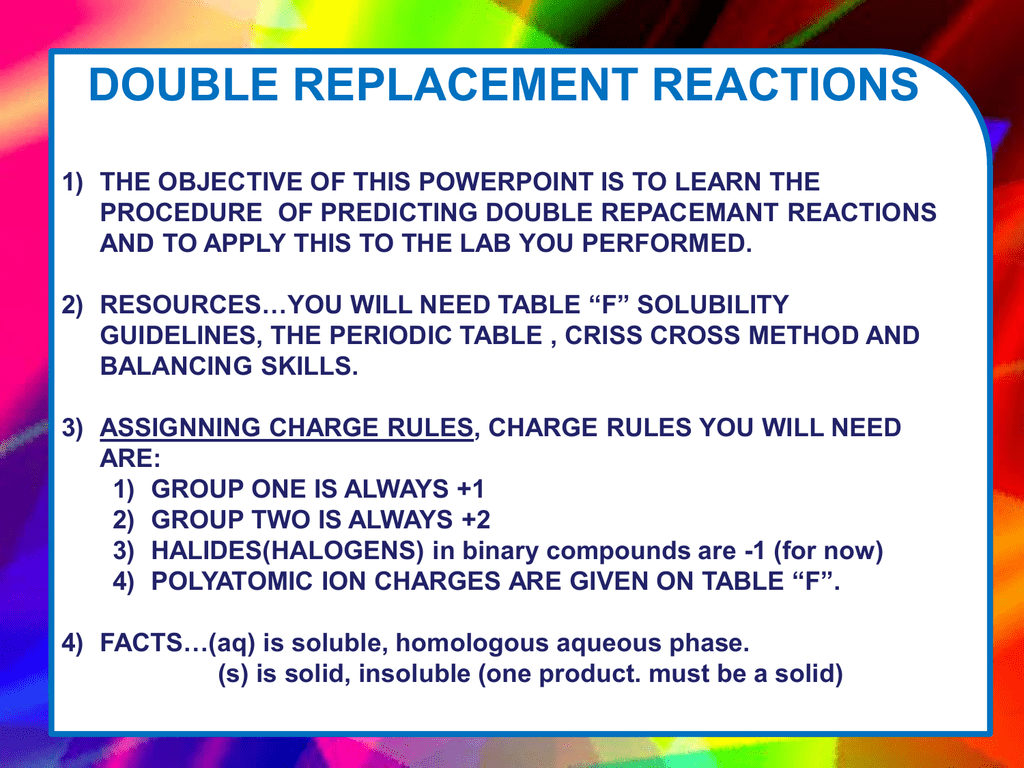



Double Replacement Reactions
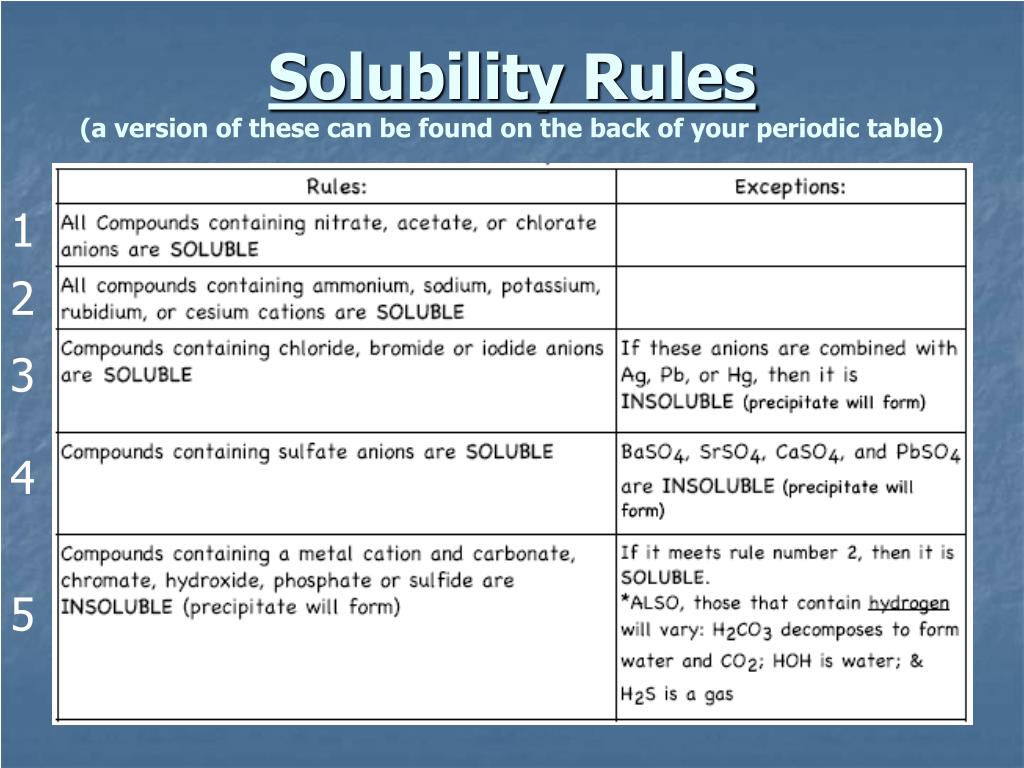



Ppt Predicting Reactions Powerpoint Presentation Free Download Id




Pin On Ap Chemistry




6 2 Precipitation Reactions Chem 1114 Introduction To Chemistry
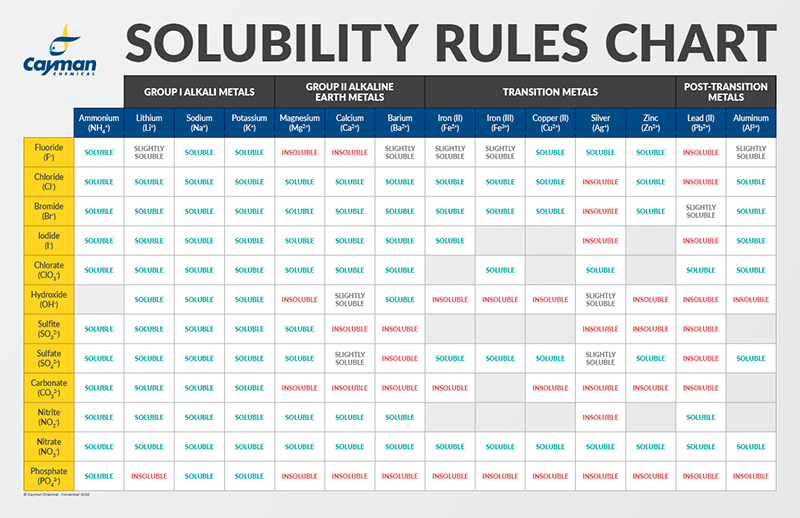



Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical



Precipitation Reactions Mrs Elliott
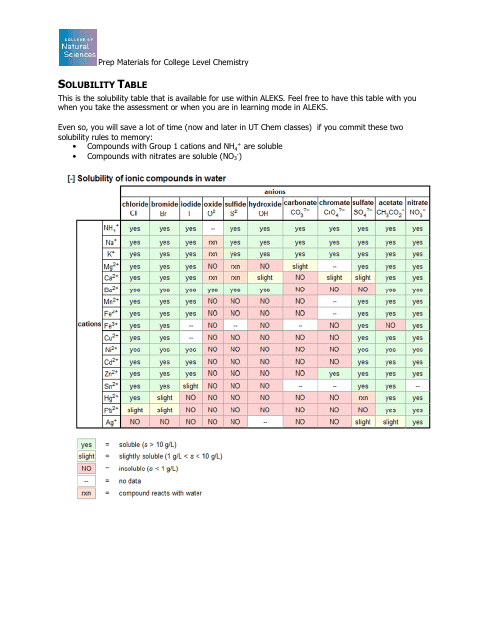



Chemistry Charts Pdf Templates Download Fill And Print For Free Templateroller
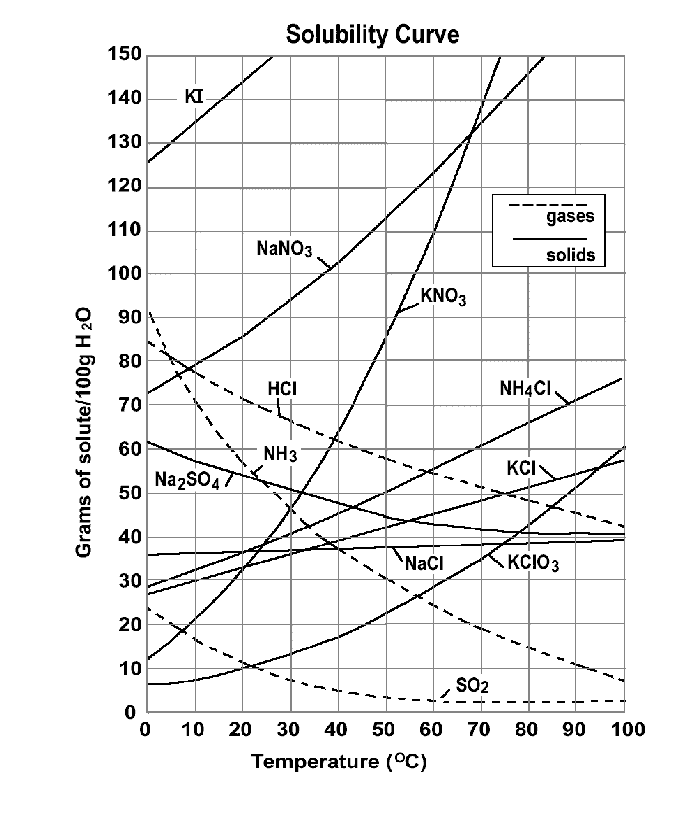



Reference Materials




Solubility Rules And Solubility Table Youtube



1




Pin On Solubility Rules Chemistry


コメント
コメントを投稿